Introduction: The HCT-CI (Hematopoietic Cell Transplantation-specific Comorbidity Index) predicts survival after HCT in patients with hematologic malignancies. HCT-CI ≥3 is associated with higher Non-Relapse Mortality (NRM) and lower overall survival in both allogeneic (allo) and autologous (auto)-HCT patients. The Disease Risk Index (DRI) Assessment Tool is a validated tool to categorize patients undergoing allo- transplant into risk categories based on disease status. It was developed to predict overall survival per disease status. This study was done to understand reasons for increased mortality in transplant patients at our center in 2021 compared to 2020 by comparing HCT-CI and DRI of transplant patients between the two years.
Methods:
The study was approved by our institution's IRB. A retrospective chart analysis of adult patients who underwent auto- or allo-HCT at Henry Ford Hospital in the years 2020 and 2021 was conducted. Relevant patient demographics, comorbidities, disease characteristics, and transplant-related data were collected. HCT-CI scores were calculated on all patients using the online calculator (https://www.mdcalc.com/calc/3980/hematopoietic-cell-transplantation-specific-comorbidity-index-hct-ci). Patients were also risk stratified based on the DRI Assessment Tool (https://cibmtr.org/CIBMTR/Resources/Research-Tools-Calculators/Disease-Risk-Index-DRI-Assignment-Tool). Data was reviewed and collected by investigators and compiled in a confidential platform.
Results:
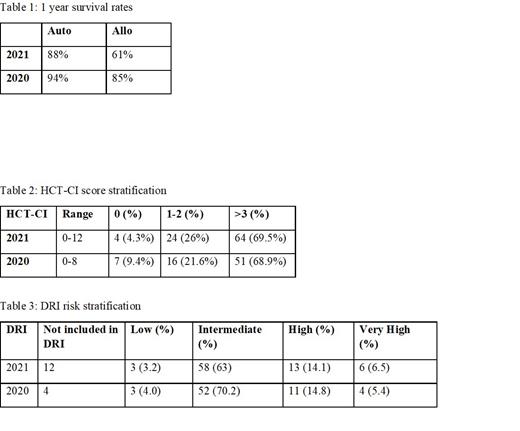
A total of 166 patient records between 2020 and 2021 were reviewed. In 2020, 46 patients underwent auto-HCT and 28 underwent allo-HCT. In 2021, 58 patients underwent auto-HCT and 34 patients underwent allo-HCT. The one-year post transplant survival rates for patients are elucidated in Table 1. The median HCT-CI scores amongst the patients who underwent transplant was 3 in 2020 and 4 in 2021(refer Table 2). When assessing the Disease Risk Index, overall, more patients with higher disease risk were transplanted in 2021 compared to 2020 (refer Table 3). 16 patients (12 in 2021 and 4 in 2020) underwent transplant for diseases that are not included in the DRI assessment tool. Of these, 8 patients in 2021 underwent allo-HCT. These included aplastic anemia (AA), inherited bone marrow failure syndromes (BMF), solid tumors, primary CNS lymphomas, mycosis fungoides and hemophagocytic lymphohistiocytosis (HLH).
Conclusion:
The HCT-CI and the DRI are both tools used in transplant centers to risk stratify patients pre-transplant. The use of both tools can give a good sense of post-transplant outcomes, combining both individual patient characteristics as well as diseases specific variations. These data are also important for monitoring outcomes at transplant centers. In this study, patients who had an HCT-CI >/3 were similar in both years. The DRI however could not be ascertained in 16 patients, as these diseases are not currently included in the DRI. This makes outcome prediction for these particular patients suboptimal especially if they do not have as many comorbidities but still higher risk diseases. Furthermore, this precludes effective outcome monitoring in and amongst transplant centers. The combination of both the HCT-CI and the DRI would perform better if the DRI tool is revised to become more comprehensive or by creating a DRI that is specific for non-malignant disorders like AA, HLA and BMF. We intend to propose this to CIBMTR for further consideration.
Disclosures
No relevant conflicts of interest to declare.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal